Linda Resar, MD, will never forget Amanda, the first leukemia patient she cared for during her oncology fellowship. The inspirational 8-year-old, who had Down syndrome, was fighting acute lymphocytic leukemia. “She was exceptionally courageous, thoughtful and resilient,” Resar said. “While she was cured and is now in her 30s, the chemotherapy was toxic, and she became ill throughout the treatment, which drives me to do better for patients like Amanda.”
Resar, a pediatric hematology/oncology specialist at the Johns Hopkins University School of Medicine, has spent the past 20-plus years trying to do just that. Now, with support from Children’s Cancer Research Fund (CCRF), she’s working to find a cure for MLL-rearranged leukemia, a particularly aggressive form of leukemia that is also known as MLL-r leukemia.
“This form of leukemia is frequently resistant to therapy and therefore associated with poor outcomes, particularly in infants. MLL-r leukemia also occurs in children and even in adults,” she noted.
Finding the "switch"
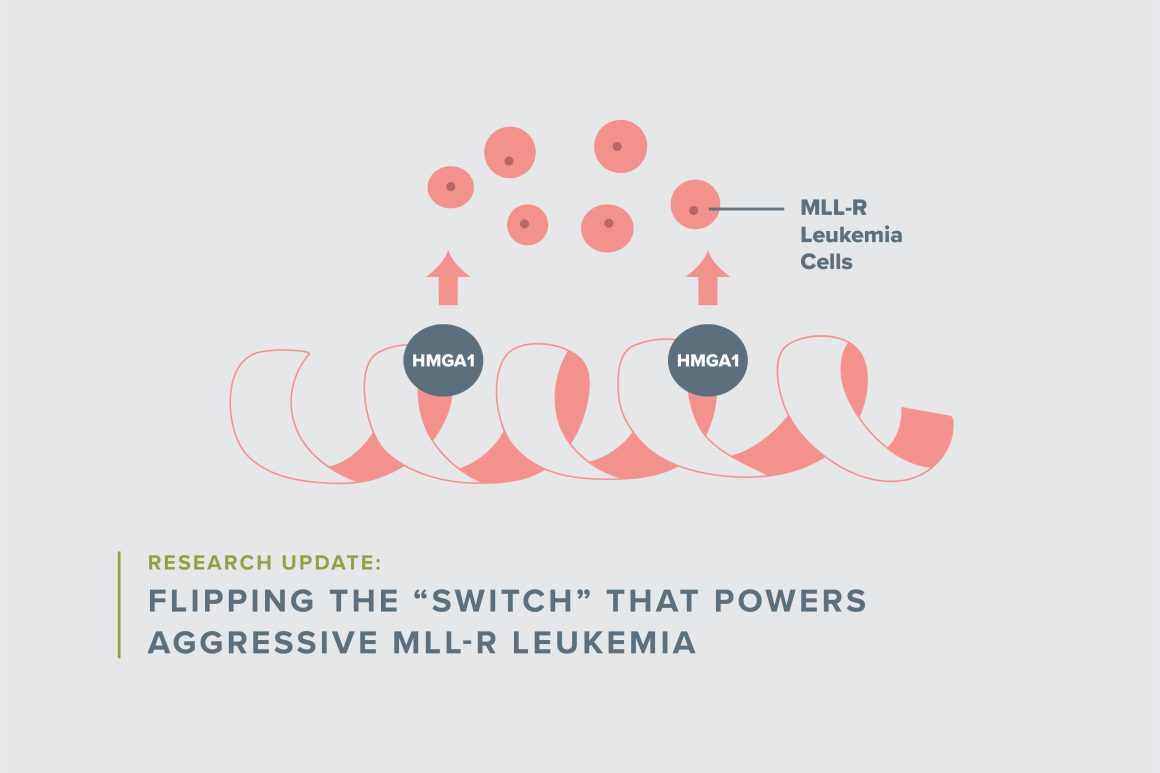
Resar’s laboratory discovered that a key regulatory protein, known as the High Mobility Group A1 protein, or simply HMGA1, is critical to the abnormal, uncontrolled growth of MLL-r leukemia cells. “Our preliminary data in leukemia cells suggest that HMGA1 functions like a light switch, flipping on genes that are required for leukemia cells to grow and to evade therapy, while turning off genes that alert the immune system to attack and eliminate the abnormal tumor cells,” she explained.
Resar initially planned to focus her research career on benign blood disorders. However, while she was training in the lab of Daniel Nathans, MD, a microbiologist at Johns Hopkins who won the Nobel Prize for his pioneering DNA research, she cloned the regulatory regions for the HMGA1 gene. Subsequent studies in her own laboratory revealed that HMGA1 functions as a potent, cancer-causing gene (or oncogene), and early studies from her group suggested that HMGA1 is activated in aggressive forms of leukemia and most diverse solid tumors. These findings sparked her interest in cancer biology and led her research team to focus on HMGA1 in childhood tumors.
“When I first started working on this gene, its function was completely unknown,” she said. “It became very clear that expression of this gene is activated in most aggressive human tumors, which suggests that it plays a fundamental role in human cancer.” In fact, the highest levels of HMGA1 are found in the most aggressive cancers, including MLL-r leukemia.
Discovering new treatment strategies
Over the years, Resar has continued to expand the field’s understanding of this pivotal gene and protein, and she is excited to build on recent discoveries. “We found that mice with a genetic deletion of one copy of this gene are healthy and make blood normally, which suggests that HMGA1 function could be blocked in therapy and tolerated without significant toxicity,” she explained. “In addition, using CRISPR technology, which allows us to edit the genome, we found that decreasing HMGA1 in childhood leukemic cells dramatically slows their growth and prevents them from forming leukemia in mouse models. Our CCRF grant is now enabling my team of dedicated scientists to investigate this process in further detail and to hopefully discover ways to block its function in therapy for our patients.”
However, targeting HMGA1 directly has proved tricky. Because it is found primarily in the nucleus of cancer cells, the compartment which houses the cells’ “genetic blue print” or DNA, therapies that specifically target proteins in the nucleus like HMGA1 had remained elusive. Now Resar and her collaborators are exploring novel strategies to inhibit HMGA1 by disrupting the protein partners or downstream pathways.
“Based on this very exciting data generated with CCRF funding, we’ve begun to work with a group that is designing ‘degraders’ for proteins like HMGA1,” she said. “Up until now, it has been extremely difficult to deliver agents or drugs that disrupt the function of nuclear proteins. We are currently designing compounds to bind HMGA1 using segments of DNA as ‘bait,’ after which the protein will be targeted to the cells’ normal machinery used to destroy proteins that are damaged or no longer needed by the cell.”
HMGA1 is also overexpressed in lymphoblastic leukemia and some forms of breast, colon, prostate, bone, skin and lung cancers, in addition to childhood tumors like neuroblastoma, osteosarcoma and brain tumors. “It has been very exciting to work on a protein that appears to be a key player in diverse tumors, and I am enthusiastic about the potential to target it in therapy,” Resar said. “Hopefully we can identify effective agents that we can bring to the clinics to help patients in the not too distant future.”
Investing in the future of cancer research
However, one of the major challenges to research in childhood cancer is funding. “Pediatric cancer research tends to be underfunded compared to research focused on adult cancers,” she noted. “Even at the National Institutes of Health, the agency that most academic laboratories rely on for support, cancer and diseases affecting children are under-served, but incredibly important. Philanthropic organizations like Children’s Cancer Research Fund are essential for projects like our research to move ahead and succeed.”
And the investment is well worth it. “In pediatric cancer, we have greater potential to cure children because they are often otherwise healthy young people with the potential for a long life ahead,” she noted.
Childhood cancer research could also help older patients. “Even in leukemia and other aggressive tumors that occur in older patients, the tumors frequently develop and progress by activating the same genes and downstream pathways that are abnormally activated in childhood tumors, such as HMGA1,” she explained. “We can learn a great deal about all tumors by studying pediatric cancers more deeply.”
Your donation makes research breakthroughs possible
By donating to Children’s Cancer Research Fund, you’re giving the brightest scientists the support they need to put their great ideas into practice. Learn more about CCRF's commitment to funding researchers early in their careers.